COVID-19 : Where does the Gut Microbiome fit in?
COVID & Gut Microbiome - what do we currently know?
SARS-Cov-2 primarily causes lung infection through binding of ACE2 receptors present on the alveolar epithelial cells
Intestinal epithelial cells particularly the enterocytes of the small intestine also express ACE2 receptors (Leung et.al., 2003)
The lungs contain their own microbiome (Zhang et.al., 2020)
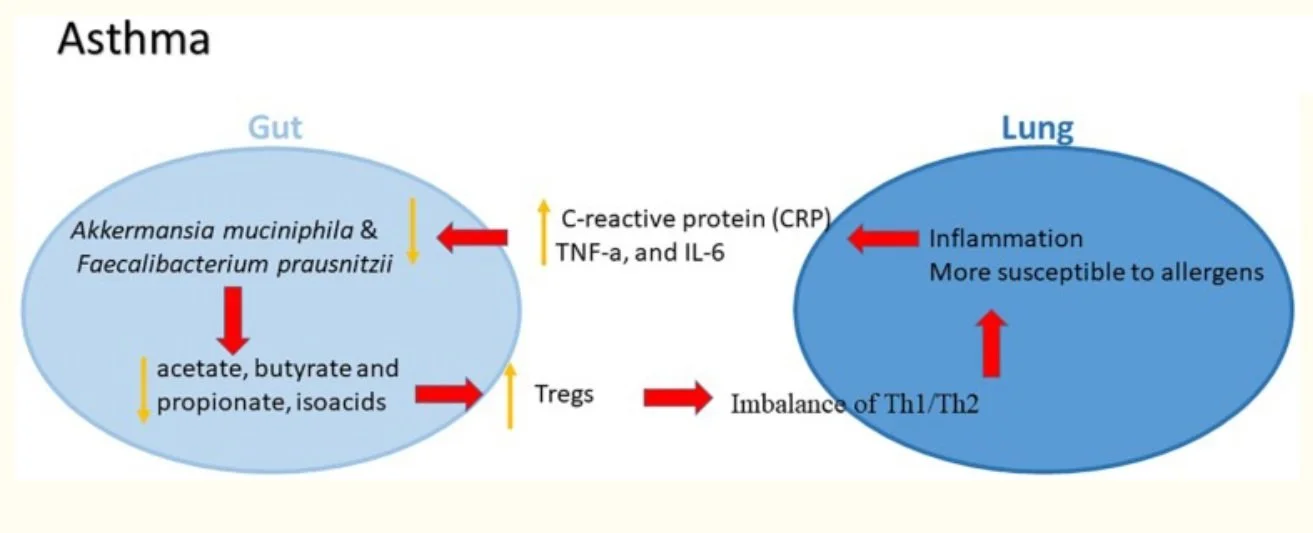
The cross talk between the gut and lungs, “Gut-Lung-Axis” is well established in the literature and the gut microbiome’s influence in lung diseases has been studied extensively. We know with Asthma for instance when compared to healthy individuals there is a tendency for decreased abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii in the gut (Zhang et.al., 2020)
Image from Zang et al., (2020) depicting The potential role of gut–lung axis in asthma.
Both Akkermansia muciniphila and Faecalibacterium prausnitzii have been shown to suppress inflammation by upregulation of anti-inflammatory Interleukin-10 (IL-10) and suppression of pro-inflmmatory Interleukin-12 (IL-12) (Demirci et.al., 2019)
Epidemiological studies have shown high fibre diets are associated with better lung function and decreased risk of Chronic Obstructive Pulmonary Disease (COPD) (Kan et.al., 2007 & Varraso et.al., 2010 ). Fibre is the fuel substrate for microbes in the gut which via fermentation stimulate beneficial microbes to produce short chain fatty acids such as butyrate. Butyrate helps maintain gut barrier integrity by serving as an important energy source for colonocytes, inhibiting the activation of NF-κB, activating the G protein-coupled receptor pair of GPR41 and GPR43, inhibiting histone deacetylase activity, which causes anti-inflammatory activities, and promoting regulatory T cells (Treg) cells (Kim, 2021)
Reduced gut bacteria diversity is associated with many diseases. The elderly have less gut bacteria diversity in general especially a decline in bifidobacterium species. (Nagpal et.al., 2018)
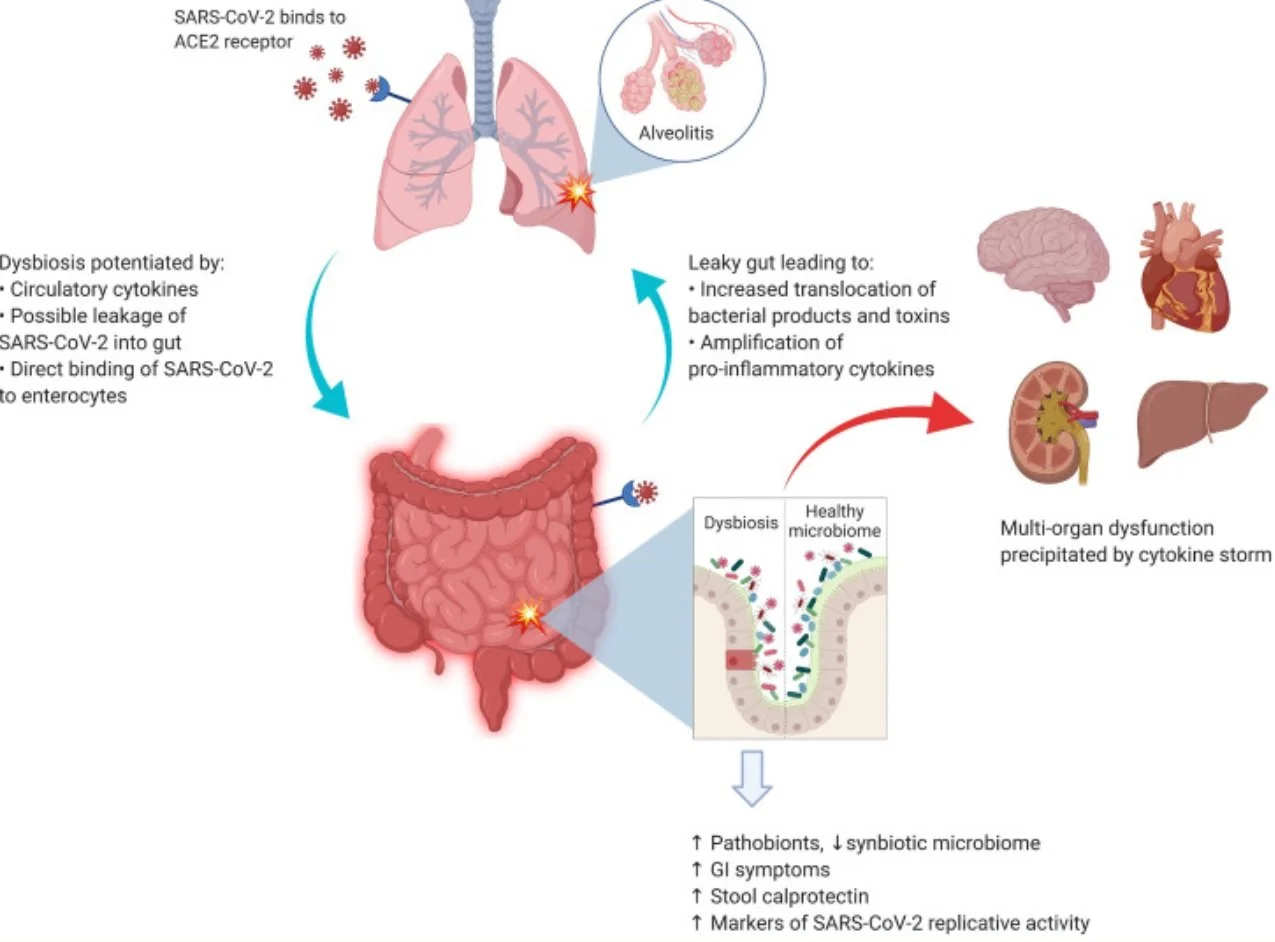
Imbalances in a beneficial vs opportunistic/pathogenic bacteria (Dysbiosis) leads to increased intestinal permeability (Leaky gut). Increased intestinal permeability then allows access to bacterial products and toxins into the circulatory system and further exacerbates the systemic inflammatory response (Hussain et.al, 2021)
Image from Hussain et.al., (2021) SARS-CoV-2 and the lung–gut axis: SARS-CoV-2 virus enters the alveolar cells by binding with ACE2 receptors, which are also abundant on the surface of enterocytes. The implication of direct infection of enterocytes by SARS-CoV-2 is still being explored. The circulatory cytokines from alveolitis (and/or direct viral infection of the enterocytes) cause the GI dysbiosis with resultant alterations in GI mucosal barrier. The entry of bacterial products and toxins from the GIT floods the circulatory system with more pro-inflammatory cytokines.
What recent studies investigating the role of the gut microbiome and its relevance to COVID-19 have brought to our attention
SARS-CoV-2 RNA has been found in the faeces of infected patients (Wu et.al., 2020). It is suggested that SARS-CoV-2 has the ability to infect and replicate in human small intestine enterocytes. (Lamers et.al., 2020).
Yeoh et.al., (2021) obtained blood, stool and patient records from 100 patients with laboratory-confirmed SARS-CoV-2 infection. Serial stool samples were collected from 27 of the 100 patients up to 30 days after clearance of SARS-CoV-2. They also investigated concentrations of inflammatory cytokines and blood markers were measured from plasma. They found significant gut microbiome composition changes in COVID-19 patients when compared to non-COVID-19 individuals. They found an underrepresentation of bacteria that have immunomodulatory properties such as Faecalibacterium prausnitzii, Eubacterium rectale and bifidobacteria in COVID-19 patients. These organisms also remained low in samples 30 days collected 30 days after disease resolution. These findings mirrored that of elevated concentrations of inflammatory cytokines and blood markers such as C reactive protein, lactate dehydrogenase, aspartate aminotransferase and gamma-glutamyl transferase. They concluded “Associations between gut microbiota composition, levels of cytokines and inflammatory markers in patients with COVID-19 suggest that the gut microbiome is involved in the magnitude of COVID-19 severity possibly via modulating host immune responses. Furthermore, the gut microbiota dysbiosis after disease resolution could contribute to persistent symptoms, highlighting a need to understand how gut microorganisms are involved in inflammation and COVID-19.”
Zuo et.al., (2020) analysed microbiome samples of 15 patients with COVID-19 and compared them to 15 healthy control individuals. Samples were collected 2-3 x per week from time of hospitalization until discharge; disease was categorized as mild (no radiographic evidence of pneumonia), moderate (pneumonia was present), severe (respiratory rate ≥30/min, or oxygen saturation ≤93% when breathing ambient air), or critical (respiratory failure requiring mechanical ventilation, shock, or organ failure requiring intensive care). They found across all time points patients with COVID-19 had an overabundance of opportunistic pathogens and an underrepresentation of Faecalibacterium prausnitzii. They concluded “In a pilot study of 15 patients with COVID-19, we found persistent alterations in the fecal microbiome during the time of hospitalization, compared with controls. Fecal microbiota alterations were associated with fecal levels of SARS-CoV-2 and COVID-19 severity. Strategies to alter the intestinal microbiota might reduce disease severity.”
In another study by Zuo et.al., (2020), they analysed microbiome samples of 30 patients with COVID-19 and compared them to 30 healthy control individuals. Samples were collected 2-3 x per week from time of hospitalization until discharge. This time they looked at the mycobiome. Patients with COVID-19 had significant alterations in their fecal mycobiomes compared with controls, characterized by enrichment of Candia albicans, Candida auris, and Aspergillus flavus. There finding paralleled those of the bacterial dybiososis patterns observed in COVID-19 patients.
Where Do Probiotics & Prebiotics Fit In?
Based off some of the recent studies that have been coming out including the ones already cited in this review there do seem to be some key findings. Decreased abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii and bifidobacteria appear to be a consistent finding with COVID-19 infection as well as lung disease in general. Interestingly these particular bacteria have immunomodulatory and antiinflammatory properties. Bifidobacteria also appear to decline with aging.
Vulevic et.al., (2015) investigated the effect of Galactooligosaccharide (GOS) supplementation in individuals aged between 65-80 and found significant increases in bifidobacteria, they also noted increased anti-inflammatory markers IL-10, IL-8 and natural killer cell activity. Thus is would seem plausible especially in the elderly to incorporate GOS supplementation as a means to improve bifidobacterium levels.
Another important key bifidogenic (bifidobacteria promoting) prebiotic fibre is xylooligosaccharides (XOS). I have done some reviews on prebiotics in previous blog posts which can be found here: Xylooligosaccharides - The quiet achiever Prebiotic, Probiotics & Prebiotics - Potential role in Athletes and Exercise, Role of the Gut Microbiome in Healthy Weight Management and Metabolic Health
As for Faecalibacterium prausnitzii don’t go rushing off to your health food store and chemist and ask for Faecalibacterium prausnitzii probiotics as they do not actually exist. Faecalibacterium prausnitzii requires specific prebiotics and the use of other probiotics to increase their numbers in the gut. Lau et.al., (2017) investigated the effects of the probiotic Bifidobacterium longum BB536 on upper respiratory tract illness in pre-school aged children. They found that Bifidobacterium longum BB536 illustrated potential protective effects against upper respiratory tract illness, they did also find supplementation with Bifidobacterium longum BB536 increased the abundance of Faecalibacterium.
Blatchford et.al., (2017) investigated the impact of supplementing with specific extracts of green (ACTAZIN™) and gold kiwifruit (Livaux™) on fecal microbial composition in functionally constipated individuals and healthy controls. In individuals who had low levels of Faecalibacterium prausnitzii to start with Livaux™ appears to stimulate proliferation of these bacteria.
Verhoog et.al., (2019) did a systematic review of the literature looking at dietary factors that impact Faecalibacterium prausnitzii. They found that inulin-type fructans, fructo-oligosaccharides, polydextrose or soluble corn fiber supplementation, and raffinose tended to increase the abundance of both Faecalibacterium prausnitzii and Akkermansia muciniphila.
Gill et.al., (2001), studied the effect of Bifidobacterium lactis HN019 ingestion in 30 elderly volunteers and showed enhanced cellular immunity as evident by Increases in the proportions of total, helper (CD4(+)), and activated (CD25(+)) T lymphocytes and natural killer cells.
Image from Villena et.al., (2020) Modulation of respiratory antiviral immunity by intestinal microbiota. Proposed mechanism for the distal immunomodulation induced by the intestinal microbiota and the enhancement of the resistance against viral infections through the improvement of the respiratory innate and adaptive antiviral immune responses.
Other worthy mentions
Quercetin has been of keen interest to researchers with several clinical studies into it’s potential role with COVID-19. I have previously written a few blog posts looking into some of the studies which you can find here:
Quercetin - benefits & what is the best absorbed form?
Vitamin C & Quercetin - potential role in the COVID-19 pandemic
Closing Remarks
So in summary I think this passage from Dhar et.al., (2020) sums things up nicely :
“Overall, it is apparent that diet mediated modulation of gut microbiota and to some extent even lung microbiota can influence immunity. Therefore, diet especially, personalized, may improve prophylaxis and can be thoughtfully administered to patients affected with Covid-19 to accelerate recovery and improve clinical outcomes.”
Some of the prebiotics mentioned can be found at the following links:
Galactooligosaccharide, xylooligosaccharides & fructo-oligosaccharide
Butyrate - Calcium/Magnesium Butyrate , SunButyrate-TG, Tesseract ProButyrate
Probiotic Strains such as Bifidobacterium lactis HN019 & Bifidobacterium longum BB536 usually require a practitioner prescription. We do offer telehealth consultations online, for more info email at info@holisticlifestyler.com
None of the products or information presented are intended to diagnose, treat, cure or prevent any disease. The information contained herein is for informational purposes only and does not establish a doctor-patient relationship.
References
Blatchford, P., Stoklosinski, H., Eady, S., Wallace, A., Butts, C., Gearry, R., Gibson, G. and Ansell, J., 2017. Consumption of kiwifruit capsules increases Faecalibacterium prausnitzii abundance in functionally constipated individuals: a randomised controlled human trial. Journal of Nutritional Science, 6.
Dhar, D. and Mohanty, A., 2020. Gut microbiota and Covid-19- possible link and implications. Virus Research, 285, p.198018.
Demirci, M., Tokman, H., Uysal, H., Demiryas, S., Karakullukcu, A., Saribas, S., Cokugras, H. and Kocazeybek, B., 2019. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergologia et Immunopathologia, 47(4), pp.365-371.
Gill, H., Rutherfurd, K., Cross, M. and Gopal, P., 2001. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. The American Journal of Clinical Nutrition, 74(6), pp.833-839.
Hussain, I., Cher, G., Abid, M. and Abid, M., 2021. Role of Gut Microbiome in COVID-19: An Insight Into Pathogenesis and Therapeutic Potential. Frontiers in Immunology, 12.
Kan, H., Stevens, J., Heiss, G., Rose, K. and London, S., 2007. Dietary Fiber, Lung Function, and Chronic Obstructive Pulmonary Disease in the Atherosclerosis Risk in Communities Study. American Journal of Epidemiology, 167(5), pp.570-578.
Kim, H., 2021. Do an Altered Gut Microbiota and an Associated Leaky Gut Affect COVID-19 Severity?. mBio, 12(1).
Lamers, M., Beumer, J., van der Vaart, J., Knoops, K., Puschhof, J., Breugem, T., Ravelli, R., Paul van Schayck, J., Mykytyn, A., Duimel, H., van Donselaar, E., Riesebosch, S., Kuijpers, H., Schipper, D., van de Wetering, W., de Graaf, M., Koopmans, M., Cuppen, E., Peters, P., Haagmans, B. and Clevers, H., 2020. SARS-CoV-2 productively infects human gut enterocytes. Science, 369(6499), pp.50-54.
Lau, A., Yanagisawa, N., Hor, Y., Lew, L., Ong, J., Chuah, L., Lee, Y., Choi, S., Rashid, F., Wahid, N., Sugahara, H., Xiao, J. and Liong, M., 2018. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Beneficial Microbes, 9(1), pp.61-70.
Leung, W., To, K., Chan, P., Chan, H., Wu, A., Lee, N., Yuen, K. and Sung, J., 2003. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection1 1The authors thank Man-yee Yung, Sara Fung, Dr. Bonnie Kwan, and Dr. Thomas Li for their help in retrieving patient information. Gastroenterology, 125(4), pp.1011-1017.
Nagpal, R., Mainali, R., Ahmadi, S., Wang, S., Singh, R., Kavanagh, K., Kitzman, D., Kushugulova, A., Marotta, F. and Yadav, H., 2018. Gut microbiome and aging: Physiological and mechanistic insights. Nutrition and Healthy Aging, 4(4), pp.267-285.
Varraso, R., Willett, W. and Camargo, C., 2010. Prospective Study of Dietary Fiber and Risk of Chronic Obstructive Pulmonary Disease Among US Women and Men. American Journal of Epidemiology, 171(7), pp.776-784.
Verhoog, S., Taneri, P., Roa Díaz, Z., Marques-Vidal, P., Troup, J., Bally, L., Franco, O., Glisic, M. and Muka, T., 2019. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients, 11(7), p.1565.
Villena, J. and Kitazawa, H., 2020. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic?. Frontiers in Physiology, 11.
Vulevic, J., Juric, A., Walton, G., Claus, S., Tzortzis, G., Toward, R. and Gibson, G., 2015. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. British Journal of Nutrition, 114(4), pp.586-595.
Wu, Y., Guo, C., Tang, L., Hong, Z., Zhou, J., Dong, X., Yin, H., Xiao, Q., Tang, Y., Qu, X., Kuang, L., Fang, X., Mishra, N., Lu, J., Shan, H., Jiang, G. and Huang, X., 2020. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology, 5(5), pp.434-435.
Yeoh, Y., Zuo, T., Lui, G., Zhang, F., Liu, Q., Li, A., Chung, A., Cheung, C., Tso, E., Fung, K., Chan, V., Ling, L., Joynt, G., Hui, D., Chow, K., Ng, S., Li, T., Ng, R., Yip, T., Wong, G., Chan, F., Wong, C., Chan, P. and Ng, S., 2021. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut, 70(4), pp.698-706.
Zhang, D., Li, S., Wang, N., Tan, H., Zhang, Z. and Feng, Y., 2020. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Frontiers in Microbiology, 11.
Zuo, T., Zhan, H., Zhang, F., Liu, Q., Tso, E., Lui, G., Chen, N., Li, A., Lu, W., Chan, F., Chan, P. and Ng, S., 2020. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology, 159(4), pp.1302-1310.e5.
Zuo, T., Zhang, F., Lui, G., Yeoh, Y., Li, A., Zhan, H., Wan, Y., Chung, A., Cheung, C., Chen, N., Lai, C., Chen, Z., Tso, E., Fung, K., Chan, V., Ling, L., Joynt, G., Hui, D., Chan, F., Chan, P. and Ng, S., 2020. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology, 159(3), pp.944-955.e8.